What Is Glaucoma?
Glaucoma is one of the leading causes of blindness.
It's caused by fluid build-up and increased pressure within the eye that damages the optic nerve that progressively worsens your vision. This disease can cause severe, irreversible vision loss if left untreated.
In many cases, appropriate control and treatment can help prevent further damage to the eyes and protect your vision. Southwest Vision Center offers several treatments to help address glaucoma. Learn more about the treatments we provide and contact us to schedule an appointment.

The 2 Main Types of Glaucoma

- Open-angle glaucoma: the most common type. It is a lifelong condition that accounts for at least 90% of all glaucoma cases.
- Angle-closure glaucoma: a rare form of the disease which requires immediate medical attention. It occurs when the angle is closed in many or most areas between the iris and cornea, which reduces fluid drainage and increases eye pressure.
Glaucoma: The Sneaky Thief of Sight
There is a reason glaucoma is called “the sneaky thief of sight”--half the people with glaucoma don’t even know they have it! Meaning, they may not have symptoms in the early stages.
At Southwest Vision Center, we diagnose and help preserve your vision with cutting-edge glaucoma treatments that can slow the progression of the disease.
If you have a family history of glaucoma or have been diagnosed with glaucoma, contact us today.

What are the Signs of Glaucoma?
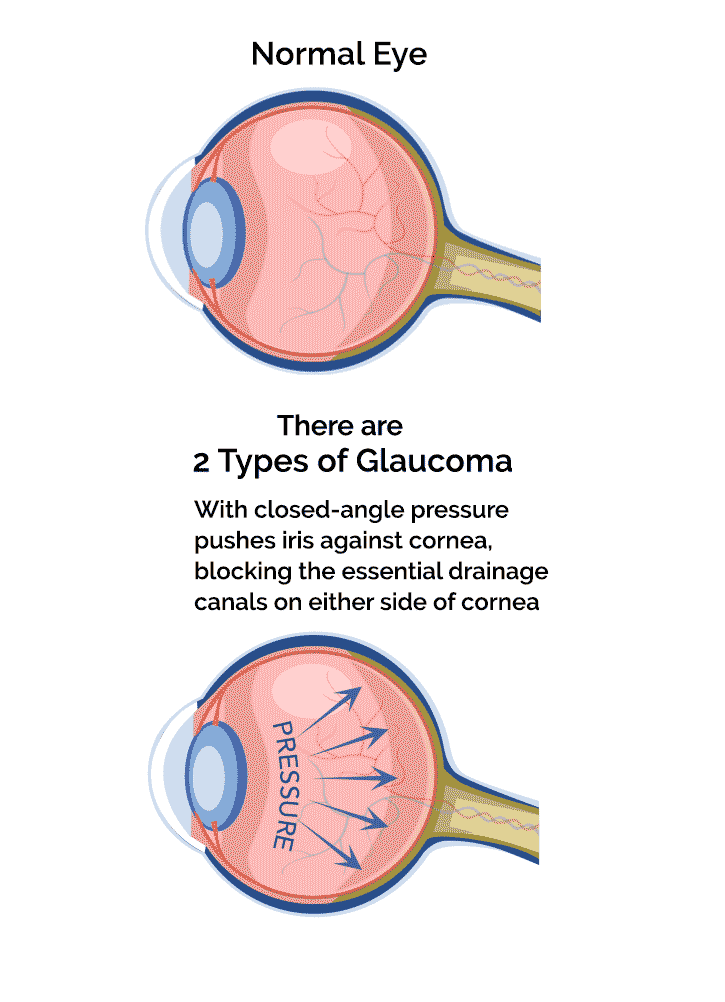
As mentioned earlier, glaucoma usually shows no symptoms in its early stages. When signs do manifest, it's usually loss of tunnel vision or peripheral vision. Unfortunately, by the time vision loss has occurred, it cannot be reversed.
That said, the less common angle-closure glaucoma does show a sudden onset of symptoms, including:
- Extreme eye pain
- Eye redness
- Blurred vision
- Nausea
If you experience any of these symptoms, seek prompt diagnosis and treatment by contacting Southwest Vision Center in North Richland Hills or visiting your nearest emergency room.
Who's at Risk for Glaucoma?
-
Age 40 +
The prevalence of glaucoma increases with age, with most glaucoma patients aged 40 and up. That said, there is a much rarer form of this disease (congenital glaucoma) that affects infants.
-
Family History & Ethnicity
Your risk for developing glaucoma is higher if you have a family history of the disease. Those of African American, Hispanic, Asian and Native American, and Indigenous Canadian descent have a higher risk of developing this disease.
-
Other Conditions
High myopia, hypertension and diabetes can increase the risk of developing glaucoma.
-
Eye Injury or Trauma
An eye injury, trauma or eye surgery can increase the likelihood of developing glaucoma.
Glaucoma Diagnosis & Treatment in North Richland Hills

Meet our Eye Doctor

- Monday 7:00 am - 5:30 pm
- Tuesday 7:00 am - 5:30 pm
- Wednesday 7:00 am - 5:30 pm
- Thursday 7:00 am - 5:30 pm
- Friday 7:00 am - 12:00 pm
- Saturday Closed
- Sunday Closed
- VSP
- Medicare
- United Healthcare
- Aetna
- Spectera
- EyeMed
- Anthem Blue Cross Blue Shield
- Blue Cross
Common Glaucoma Treatments
There is currently no cure for glaucoma. However, several treatments are available to prevent the progression of this sight-robbing condition. With the right care, you have a higher chance of managing your glaucoma and preserving your vision.
Common treatments include:
Eye Drops
Your optometrist will prescribe eye drops to help regulate pressure inside the eye by decreasing fluid production and/or improving drainage.
Laser Surgery
If eye drops aren’t doing enough to reduce intraocular pressure or intraocular temperature in open-angle glaucoma, laser surgery may be an option. Selective laser trabeculoplasty (SLT) opens up the drainage system in the eye to reduce pressure.
SLT is successful in 80% of cases and can reduce eye pressure by 20%.
Other Surgeries
If a laser procedure or eye drops do not lower eye pressure to the desired level, your eye doctor may recommend one of the following surgeries:
- Incision Surgery
- Minimally invasive glaucoma surgery (MIGS)
- Glaucoma Drainage Implants
Glaucoma Testing Treatment FAQs
Since glaucoma has no side effects early on, it is essential for people with a family history of risk factors for glaucoma to get tested. The following tests diagnose glaucoma:
- Tonometry: measures the pressure inside the eye (intraocular pressure or IOP)
- Ophthalmoscopy (dilated eye exam): examines the shape and color of the optic nerve
- Perimetry: measures your field of vision
- Gonioscopy: checks the angle where the iris meets the cornea
- Pachymetry: determines the thickness of the cornea to better evaluate eye pressure.
The following are the foods every glaucoma patient should consider avoiding immediately. It should help keep your optic nerve healthy and minimize eye pressure.
- Caffeine - certain studies show that caffeine contributes to increased intraocular pressure.
- Saturated fats - a diet high in saturated fats can lead to weight gain, which not only increases intraocular pressure but also cholesterol levels.
- Trans fats - try to limit your consumption of trans fats because they can also raise cholesterol levels
- Salt - make sure to consume salt sparingly, as increased blood pressure can indirectly lead to intraocular pressure.
About 60% of patients diagnosed with glaucoma will eventually lose some vision.
However, the rate of legal blindness among glaucoma patients is 5%. Getting effective treatment early on will greatly increase your ability to preserve and maximize your vision.